My Treatment Plan
After completing the imaging tests, surgical biopsy and bone marrow biopsy, I was diagnosed with Stage 3A Nodular Sclerosis Hodgkin Lymphoma.
I was Stage 3 because my final staging PET scan showed there was cancerous lymphatic activity in my spleen. While there are no lesions or any lymphatic involvement below my diaphragm and by labs and blood levels look relatively normal, my doctors decided to treat me as Stage 3 to ensure that they would effectively kill all microscopic traces of the lymphoma in my system.
The standard course of treatment for Stage 3 Hodgkin Lymphoma is 6 cycles of ABVD chemotherapy followed by radiation and a stem cell transplant if it does not respond to chemotherapy. The length of treatment is approximately 6 months. Typically, this type of chemotherapy is very effective at killing malignant cancer cells
Each cycle of ABVD is 28 days or 4 weeks.
Here is a breakdown of the ABVD regimen:
One cycle of ABVD chemotherapy is typically given over 4 weeks, with two doses in each cycle (on day 1 and day 15). All four of the chemotherapy drugs are given intravenously. ABVD chemotherapy is usually given in an outpatient setting and does not require hospitalization.
Typical dosages for one 28-day cycle of ABVD are as follows:
I signed up for a clinical trial at Northwestern where I received 3 cycles of immunotherapy prior to starting chemotherapy. I also had a power port surgically inserted into my chest to help make the chemotherapy infusions easier and help preserve my veins during the course of treatment. It made the whole treatment process easier overall so I am very happy with my decision.
My treatment varied slightly from the standard approach. They removed the "B" drug known as Bleomycin because it is the most toxic of the four chemotherapy drugs used to treat lymphoma. After I completed 3 cycles of immunotherapy, I started the AVD regimen of chemotherapy to target the remaining lymphoma cells.
Immunotherapy Results
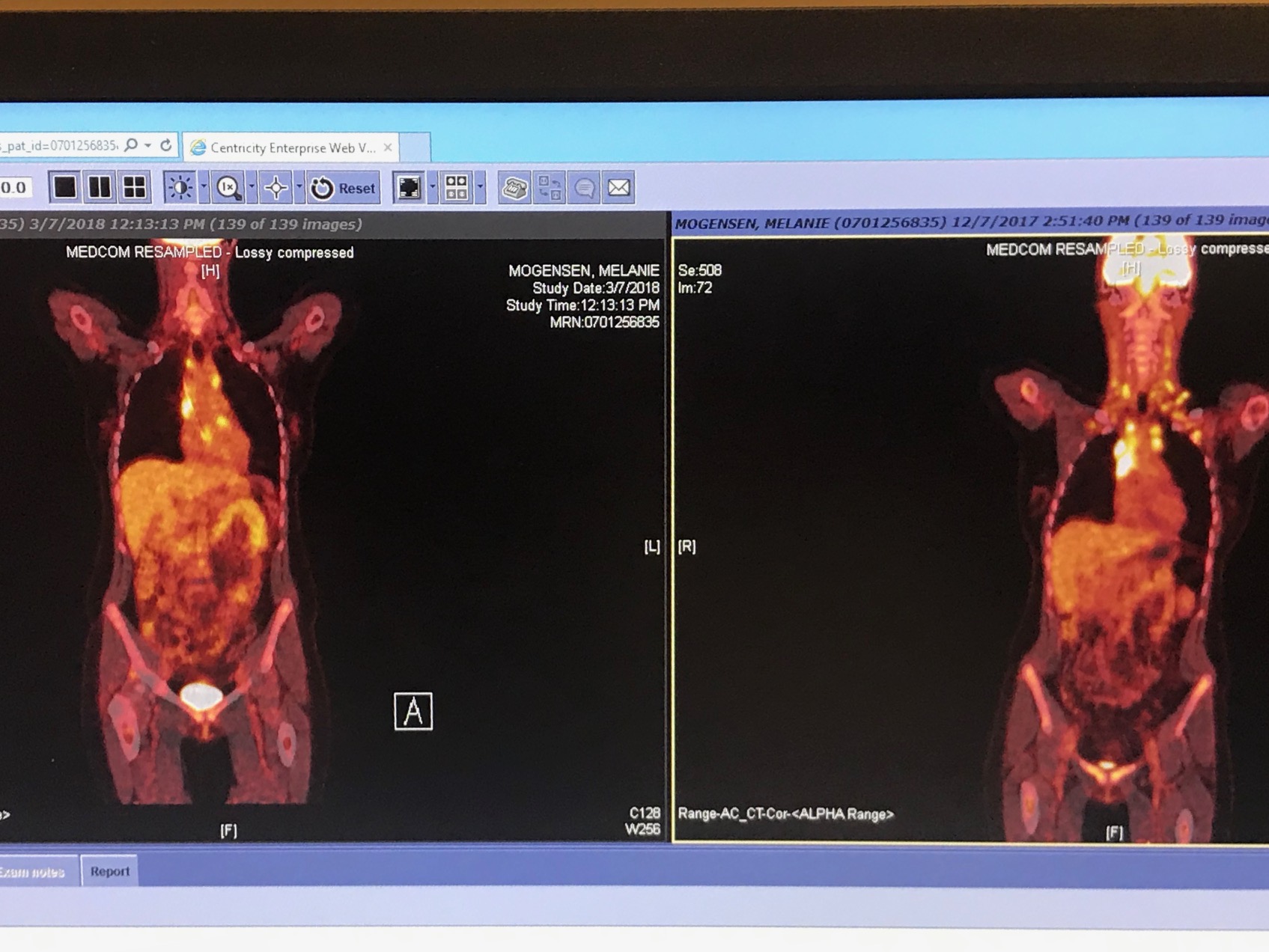
I finished the three immunotherapy treatments in February. In early March, I had a follow up scan to show the effectiveness of the immunotherapy. I'm so happy to report the scans showed the immunotherapy treatment was incredible effective! I could tell I made a significant improvement before the scans just by how great I was feeling. I had a huge increase in energy and overall stamina. Sure enough, the scans showed all the cancerous activity in my neck was GONE! I just had a little activity in my chest area that the (AVD) chemotherapy would hopefully clear.
The scan on the left is current state as of 3/8/18. The previous scan on the right was taken in late December 2017. In just three months of immunotherapy treatment, you can see a huge difference in my neck region (on left) I also had some cancerous activity in my arm pits and spleen which is also GONE! Just some slight cancerous activity remains in my chest. Very exciting results!
I began my first round of chemotherapy on March 8, 2018. In total, since I am Stage 3, I had six cycles of AVD chemotherapy which equaled 12 individual treatments.
Overall, I tolerated chemotherapy pretty well. I just experienced some fatigue, hair loss and nausea. I like to compare chemo to the feeling of early pregnancy. Having been through two pregnancies (one being a twin high risk pregnancy) I definitely feel they are quite similar!
I have also started to feel BETTER during the course of chemotherapy which surprised me. Following the journey of others with Lymphoma, I know that this is not always the case, so I really feel that the immunotherapy is a huge part of my treatment success.
Incredibly, I found out on May 1st, 2018 during my midway scan that there was No Evidence of Disease. Miraculously, I was able to reach remission from Stage 3 Hodgkin Lymphoma after just three months of immunotherapy and two cycles (four individual treatments) of chemotherapy.
Although my scan showed no evidence of disease midway through chemotherapy I still had to finish all six cycles (12 treatments) to ensure I had the best chance at long-term remission.
On August 9th 2018, I finished chemotherapy. On September 27th, I had a repeat PET scan. The scan showed a reactive node in my stomach. I required an additional CT scan to rule out a relapse; however on October 1st my scan showed the node was benign so I am still in REMISSION! I will continue to be monitored closely and will have repeat scans every 3-6 months for the next year.
Update:
I had the power port in my chest removed and my last routine CT scan on September 14th, 2020 since finishing treatment in August of 2018. Everything looked great on my scans so I was told I no longer need to be monitored as closely with visits or scans. According to my doctor, two-years cancer-free from lymphoma is a huge milestone in remission because now my risk of relapse is about the same as the general population.
Immunotherapy Clinical Trials
If you or your loved one was recently diagnosed with Lymphoma, I urge you to consider a combination of immunotherapy and chemotherapy. I believe the immunotherapy was instrumental in me going into remission, just 5 months after being diagnosed as Stage 3.
Here is more information on the clinical trial I was a part of at Northwestern:
NU 16H08: Phase II study of PET-directed frontline therapy with pembrolizumab and AVD for patients with classical Hodgkin lymphoma
The purpose of this research study is to evaluate a new drug, pembrolizumab, followed by chemotherapy, for the treatment of newly diagnosed classical Hodgkin lymphoma. The chemotherapy regimen is called “AVD” and includes three drugs: doxorubicin, vinblastine, dacarbazine. Pembrolizumab is currently FDA approved for the treatment of relapsed Hodgkin lymphoma, but is not approved for use in newly diagnosed patients. The ‘ABVD’ regimen of chemotherapy is the standard of care for the treatment of newly diagnosed classical Hodgkin lymphoma. Not all patients achieve a complete remission with ABVD, and older patients tolerate the regimen poorly. Patients who do not have a complete response to standard ABVD chemotherapy (meaning there is still evidence of disease on PET scans performed at the end of treatment), may be treated with radiation or a more intensive chemotherapy regimen. Patients who don’t achieve a complete remission with frontline treatment (refractory disease) or those who relapse require treatment with high dose chemotherapy and a stem cell transplant. Older patients, however, are not candidates for intensive chemotherapy or stem cell transplant. This is a phase II study of pembrolizumab followed by AVD (ABVD without bleomycin) for patients with newly diagnosed classical Hodgkin lymphoma. Some older patients may receive an additional one to two years of pembrolizumab maintenance.
Eligibility CriteriaSome of the eligibility criteria include:
• Patients must have a confirmed diagnosis of classical Hodgkin lymphoma.
• Patients must have previously untreated disease
• Patients must be 18 or older.
Note: This is only a partial list of eligibility criteria. Please contact the Robert H. Lurie Comprehensive Cancer Center of Northwestern University for complete screening information if you are interested in this clinical trial.
Principal investigator: Dr. Jane Winter
http://www.feinberg.northwestern.edu/Research/clinical-trials/trials.html